FDA Approves Marketing of First mHealth Solution for Contraception
The app is driven by an algorithm that combines body temperature input with menstural cycle data.
The US Food and Drug Administration (FDA) has approved the marketing of the first mHealth solution for contraception — a mobile app called Natural Cycles that allows women to avoid pregnancy by a method known as fertility awareness.
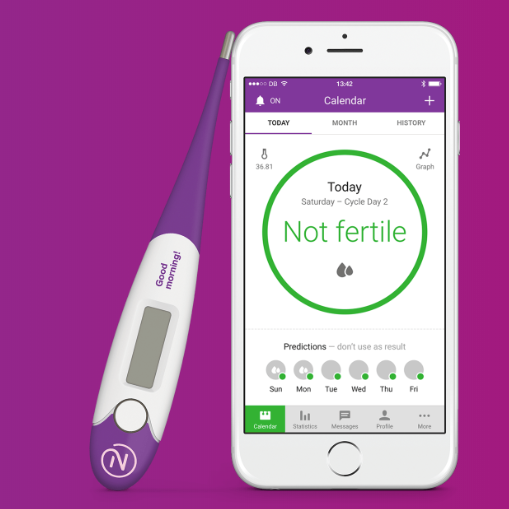
The Natural Cycles app is driven by an algorithm that can calculate which days of the month a woman is likely to be fertile based on a combination of menstrual cycle monitoring and daily basal bodily temperate input. Basal body thermometers can detect minor rises in body temperature of about half a degree Fahrenheit around the time of ovulation, which signify to the user that they are at peak fertility (typically between 4 to 5 days per month).
“Consumers are increasingly using digital health technologies to inform their everyday health decisions, and this new app can provide an effective method of contraception if it’s used carefully and correctly,” said Terri Cornelison, M.D., Ph.D., assistant director for the health of women in the FDA’s Center for Devices and Radiological Health. “But women should know that no form of contraception works perfectly, so an unplanned pregnancy could still result from correct usage of this device.”
The marketing approval is based on clinical studies of Natural Cycles that included more than 15,000 women. The app had a “perfect use" failure rate of 1.8%, which means that 1.8 in 100 women who use the app for one year will become pregnant because they had sex on a day when the app predicted they would not be fertile, or because supplemental contraceptive methods failed during intercourse on a “fertile day.”
Natural Cycles had a “typical use” failure rate of 6.5%, which accounted for women sometimes not using the app correctly, for example, by having unprotected sex on fertile days.
The FDA has warned that Natural Cycles should not be used by women who have a medical condition where pregnancy would be associated with a significant risk to the mother or the fetus, or those currently using birth control or hormonal treatments that inhibit ovulation. Additionally, Natural Cycles does not provide protection against STIs.
The approval comes after the FDA released the Digital Health Innovation Action Plan last year, to increase their scrutiny and attention of digital health technologies.
Related >>
A High-Tech Day for the FDA Emphasizes Its Digital Shift
FDA Releases Updated Software Precert Model
FDA Announces New Device Guidance, Enanced Patient Input Program