New robotic surgical device now being used in hospitals
Memic Innovative Surgery, an Israeli-based company focused on robotic surgical technology, touts its new device for gynecological procedures. The Hominis Surgical System is now in use at three hospitals.
A new surgical robot appears to be on the cutting edge.
Memic Innovative Surgery said last week its new robotic surgical system for gynecological procedures is now in use at three top hospitals. Memic, based in Israel, focuses on robotic surgical technology.
Last year, the U.S. Food and Drug Administration authorized marketing of Memic’s Hominis Surgical System. The FDA said the device offers a minimally invasive option for gynecological procedures. The FDA said it is the first robotically-assisted surgical device approved for a transvaginal hysterectomy.
Dvir Cohen, co-founder and CEO of Memic Innovative Surgery

Memic announced the system is now being used at three Florida hospitals: HCA Florida Healthcare’s Kendall Regional Medical Center, AdventHealth Celebration and The Women’s Hospital at Jackson Memorial. While Memic is based in Tel Aviv, the company has a subsidiary based in Fort Lauderdale, Fla.
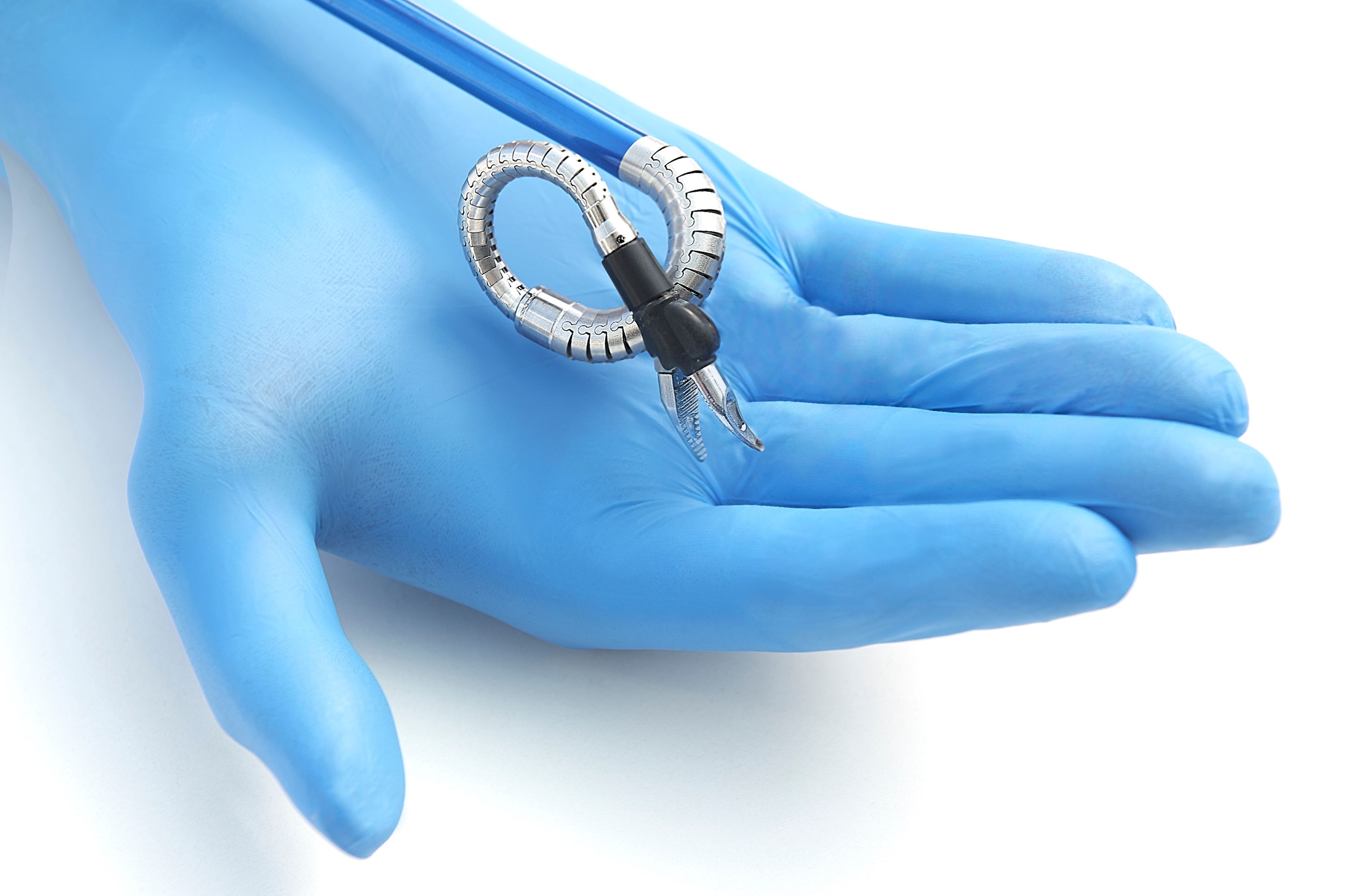
The company said the surgical robot is designed to replicate the motions of a surgeon’s arms. The device “features miniature humanoid-shaped arms, with shoulder, elbow, and wrist joints that provide human level dexterity and 360-degree articulation,” Memic said in a news release.
The robotic device still requires a doctor at the controls.
The Hominis system uses minimally invasive instruments inserted through the vagina. A video camera is inserted through a small incision in the abdomen to allow the surgeon to see the instrument. Since the approach requires fewer incisions, there is less scarring, allowing patients to recover more quickly, the company said.
Memic also said Hominis is less expensive than other robotic systems, allowing more hospitals to acquire the device.
Memic Innovative Surgery, based in Israel, said its Hominis Surgical System is now being used in three hospitals. The device is used for minimally invasive gynecological procedures. (Image submitted by Memic.)
Dvir Cohen, co-founder and CEO of Memic, said the adoption of the Hominis system in the three hospitals is a step toward expansion worldwide.
“We are proud to partner with these leading healthcare providers to empower surgeons with a better user experience and help them better serve the thousands of women who undergo gynecologic surgery each year,” Cohen said in a news release.
Leaders of the three hospitals that are using the Hominis system offered glowing praise. Ricardo E. Estape, a gynecology oncologist at Kendall Regional Medical Center and director of HCA Healthcare’s Institute for Gynecologic Oncology, said he appreciates being among the first hospitals to use the device.
The system allows the hospital “to perform minimally invasive gynecological procedures using the transvaginal approach, which is known to offer less pain, lower infection rates, faster recovery, and no visible scarring,” he said in a news release.
Doug Harcombe, CEO at AdventHealth Celebration, said the hospital welcomed the chance to add new technology to surgical options. “This technology will allow us to perform surgical techniques not feasible today and improve the surgical experience for a wide range of patients,” he said in a statement.
“As one of the region’s most cutting-edge surgical centers, we are excited to be adding new technology and surgical solutions that will benefit our patients,” said Joanne Ruggiero, interim CEO of Holtz Children’s Hospital/The Women’s Hospital at Jackson Memorial.
The FDA said it reviewed the device through the De Novo pathway, which is designed for new products with low to moderate risk. In its review, the FDA evaluated a clinical study of the Hominis system with 30 patients, and the agency said all the procedures were done successfully.
Memic is in the process of merging with the MedTech Acquisition Corporation, based in Connecticut. The merger is expected to be completed in the first quarter of 2022. The combined company will be known as Memic when the deal is complete.
The company has applied to list its shares on the Nasdaq Stock Market.
Telehealth faces a looming deadline in Washington | Healthy Bottom Line podcast
February 12th 2025Once again, the clock is ticking on waivers for telemedicine and hospital-at-home programs. Kyle Zebley of the American Telemedicine Association talks about the push on Congress and the White House.